Solutions are customized to suit the specific needs of our clients
Inventory & Master Data Mgmt.
- Benchmark inventory levels, set up process of re-ordering, auto replenishment, consumption
- Track utilization & spend on LAB consumable assets
- Work out more inclusive consumption based costing for all tests
- Track expiry, return to vendor, contract mgmt.
Instrument & equipment Qualification:
- Continuous update on all sites instrument’s – upgrade / retiral plan & ensure high availability
- Deliver holistic service pack comprising SOP, Validation, Training, Testing, re-validation and awareness program
- Track inter-dependencies viz. application, infrastructure, hosting, methods & deliver
Calibration & Preventive maintenance:
- Plan & execute scheduled calibration & Preventive plans – Display & deliver efficiency
- Track efficient utilization of assets
- Establish Best practice of documentation
- Assist Management team on overall resource optimization – People & instrument through process digitization
Laboratory Planning & Program Mgmt.
- Plan LAB throughput based on rolling forecast of production plan, RM delivery, stability, maintenance, people competency and instrument availability
- Track week on week actual vs. Planned , report & rework to attain target
- Implement Agile Methodology
Stability Management
- Program to manage the entire Stability Sample /Chamber management, retrieval , reconciliation & retention
- Planning & resource allocation
- Ensure Stability schedule adherence & regulatory compliance
OOS/ OOT/ Deviation - Failure investigation and redressal
- Comprehensive investigation into OOS / OOT trend and predictive analysis using Six Sigma methodology
- Corrective & Preventive advocacy
- Monitor metrics for the future improvisation
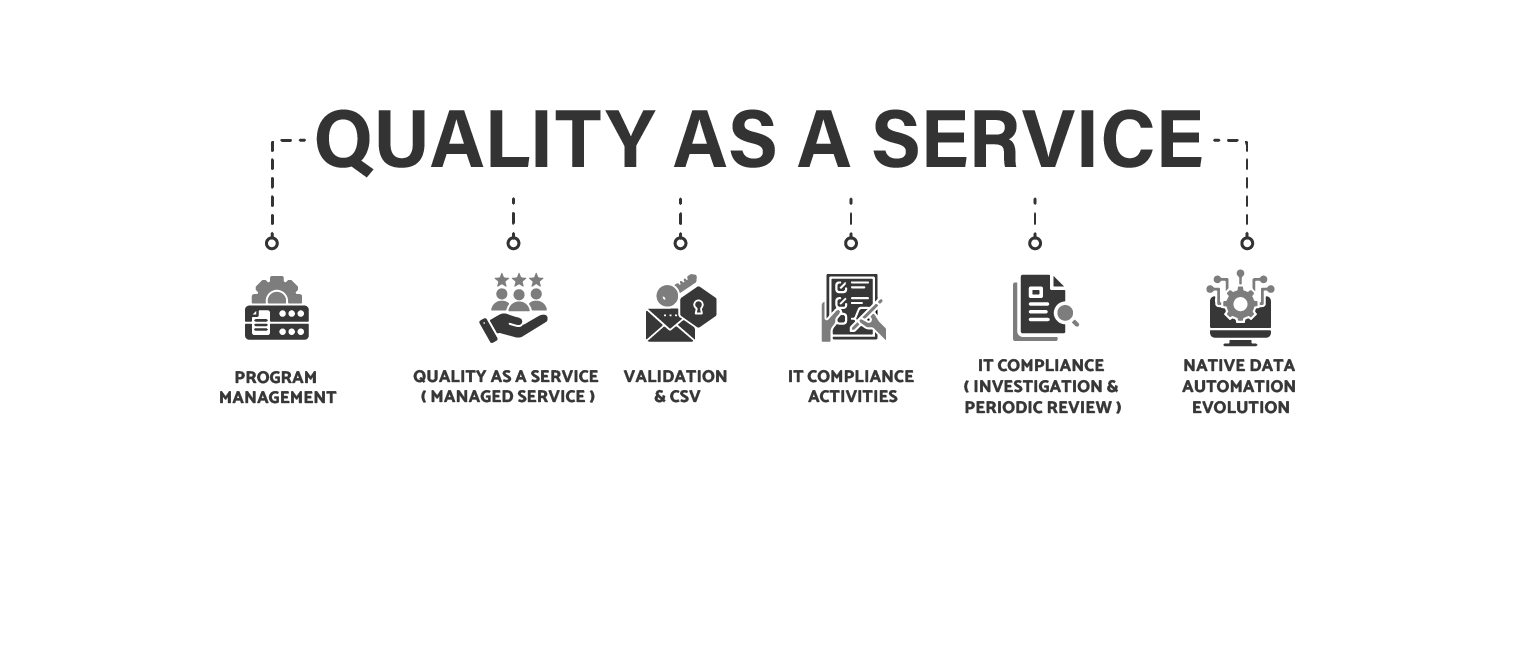
5 Global Principles of our Program Management
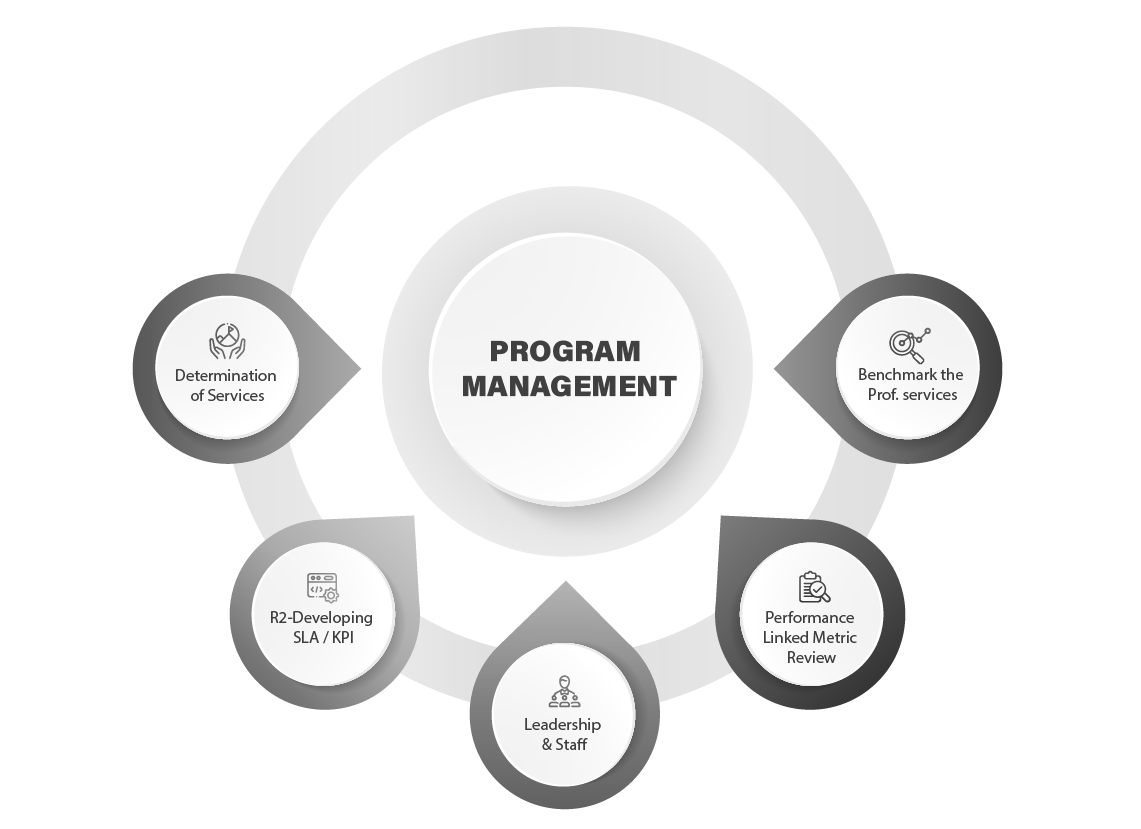
R1-Determination of Services
- Detailed "Operating Book" creation for each function within the LAB with defined SLA's
- All the activities on the floor and there dependencies will be mapped to measure & monitor progress and/or impediments
R2-Developing SLA / KPI
- Create a transparent partnership between line and staff services to set the correct expectation between demand & supply
- SLA's, KPI's, Charge backs / penalties for not meeting SLA's / KPI's would be drawn up in consultation
R3-Leadership & Staff
- Subject matter experts & advisory team of Synthesis will have high focus towards efficient service delivery mindset
- Transition management process orientation would be the 1st step to bring Customer expectation in the entire program
R4-Performance Linked Metric Review
- Each of the Service units are assessed based on the KPI / SLA based review.
- Performance linked incentive plan will be specific to each vertical for the specialized services rendered.
R5- Benchmark the Prof. services
- Performance indicators are identified, tracked based on the Industry standards.
- External/internal benchmarks and best practices are monitored by Synthesis Governance Council.
Qaas - Validation & CSV
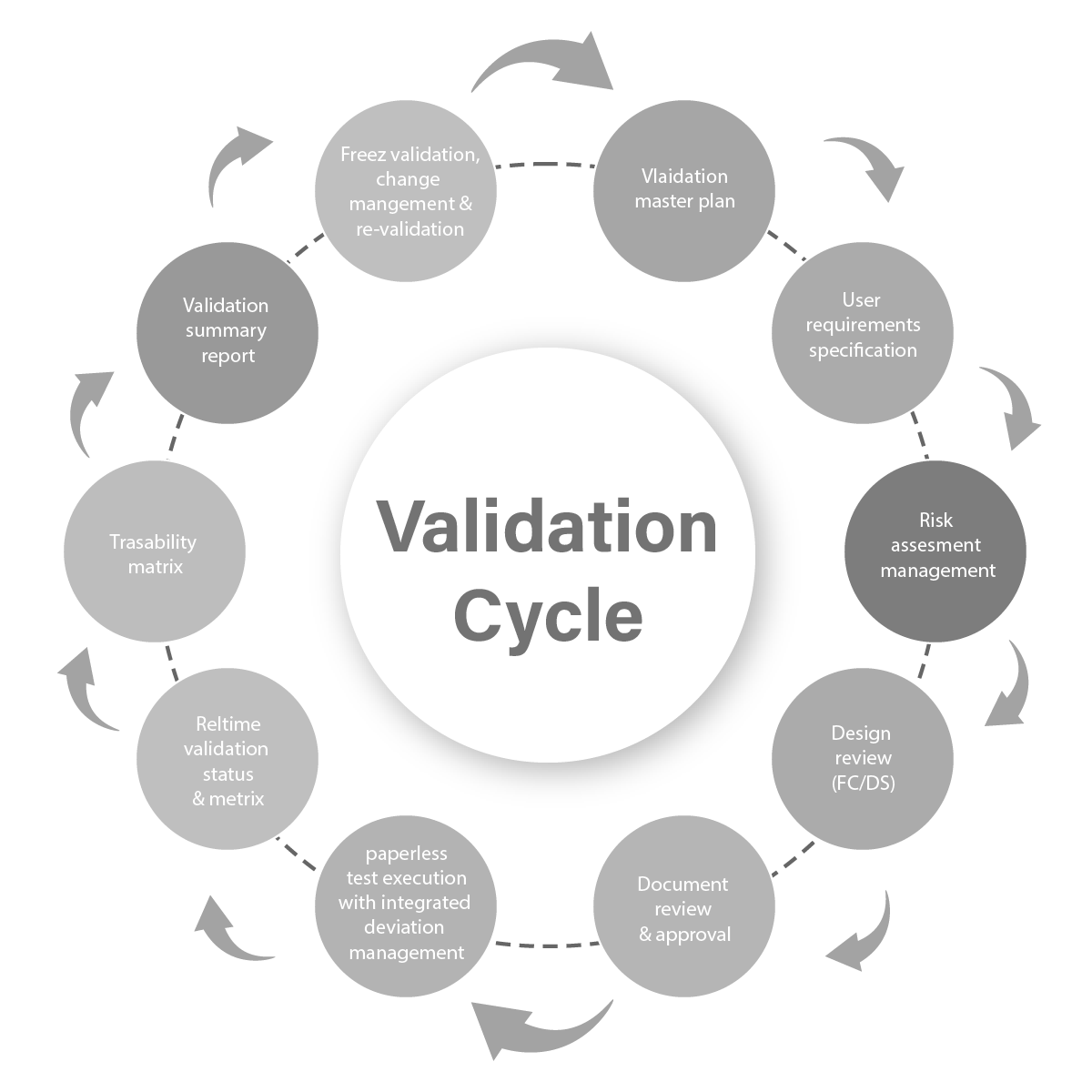
Our Collective experience span across the domain of Life Science addressing the entire spectrum of the “Validation Life Cycle Management” for Instruments / Equipment’s – Commissioning & Qualification and CSV activities.
FAT / SAT review for the Equipment’s / Instruments - Categorization of the systems as per GAMP & Assessment (21 CFR Part 11, EU Annex 11, USP 1058, etc.,) - Impact & Risk Assessment Documentation for Mitigation of the GAP’s / Risk’s - Validation plan & Strategy Design - Development of Validation documentation - Execution of the Validation actions & Reporting
Change Management
- Change Control Management
- CR Approval workflow, Tag CR to the projects
Impact Assessments
- based on GxP checklists
- GAMP categorization of system
- FMEA based risk assessment
Validation Documentation & Execution
- URS, FS, DS, Risk Management, Requirement Traceability, Test case setup & execution
- Review & Approval Workflow
- Periodic Reviews

IT Compliance Areas

Managed Services comes under the Strategico Governance structure where specialists execute the defined activities and maintain consistent state of readiness as an extended arm of the client to ensure Zero deficiency in Compliance to IT Laboratory & Manufacturing Compliance areas
Current State Assessment of the Existing Systems, operations, Vendor Management, Validation Status, etc.,
Mitigation action of Process, Procedure if any. Plan for Transition with Defined scope for User Management, Incident Management, New Installation , etc.,.
Implementation of QaaS for the 1st site with all the aligned actions & job role. Scheduled Governance Review on periodic intervals
IT Compliance Activities
User Management & Periodic Review
Documentation of the User creation, User deactivation, role change, etc., Preparation of the Privilege Matrix, Periodic review of the Active users & Privileges.
Lab / Mfg. Systems backup & Restoration
Maintenance of the Backup system list, Documentation of Restoration calendar, Periodic review of the Backup Process & Restoration.
Upgrade / Reinstallation, Re-validation of Systems
Upgrade of the Instrument / Equipment software Vendor Coordination, Support on Documentation relation to Upgrade & Reinstallation on Risk assessment & QMS events, etc.,
GxP Documentation, Audit Support
Documentation of System Retirement, Upgrade & SOP Documentations. Training on the SOP’s to Business users, Support during the Regulatory & Customer audit
Periodic Re-validation & CS Validation execution
Performing the Re-Validation of the systems as per the Periodic review calendar & Execution of validation for systems in case of the Revalidation, upgrade, Data Verification as part of investigations, etc.,
IT Compliance ( Investigation & Periodic Review )

Document articulation and review
- Technical writing
- Investigation process review
Robust Investigation process & coaching
- Coach & establish robust a Investigation process using Six Sigma tools
- CAPA effectiveness check
- No-repetitive RCA by addressing the systemic cause for failures
- Thorough documentation and Regulatory compliance
Audit support & Data Management
- Investigation proofing Document error
- Data trending & mitigations
- All time readiness w.r.t & Documentation availability
- Vendor Coordination & Support

Robust failure Investigation solutions
- Investigation process establishment & hand
- Investigation process that can be applied to Laboratory and manufacturing shop floor activities.
- Addresses the causes of failures holistically.
- Detailed Documentation and Data analysis.
- CAPA sustenance & Root causes ensured to be non-repetitive.
- Better Regulatory compliances & confidence.
- Increases 'Quality deliverables on time with elimination of non value added activities